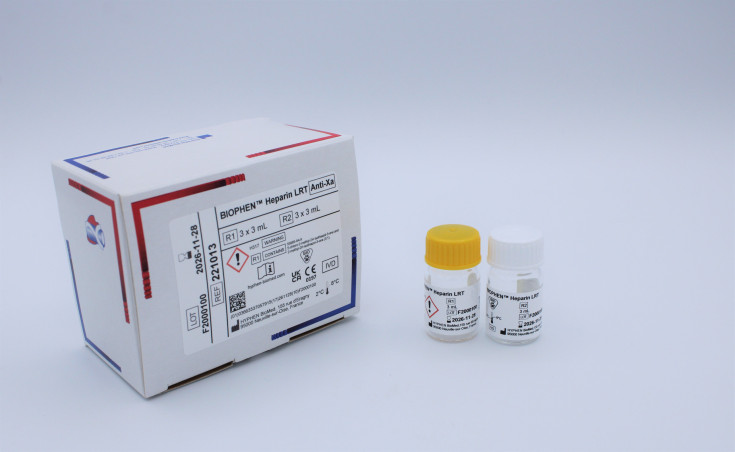
BIOPHEN™ Heparin (LRT)
BIOPHEN™ Heparin (LRT)
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
An anti-Xa chromogenic method for quantitative determination of Factor Xa inhibitors in human plasma for monitoring anticoagulant therapy. For in vitro diagnostic use.
Product Overview:
BIOPHEN™ Heparin LRT is an anti-Xa chromogenic method for the in vitro quantitative determination of Factor Xa (FXa) inhibitors in human citrated plasma. It is intended for monitoring patients on Heparin (UFH/LMWH), Arixtra®, or Orgaran® therapy, and for specific clinical situations involving oral anticoagulants such as Apixaban, Rivaroxaban, and Edoxaban. In vitro diagnostic kit for professional use only.
Principle and Application:
This method is a one-stage chromogenic assay based on the inhibition of a constant, excess amount of Factor Xa by the anti-Xa substance in the sample, in the presence of endogenous antithrombin (AT). The residual FXa then hydrolyses a specific chromogenic substrate, releasing para-Nitroaniline (pNA). The amount of pNA released, measured by absorbance at 405 nm, is inversely proportional to the concentration of the anti-Xa inhibitor in the sample.
Product Composition and Specifications:
This kit contains liquid, ready-to-use reagents.
- R1: Chromogenic substrate specific for Factor Xa (CS-11(32)) at approximately 2 mg/mL.
- R2: Bovine Factor Xa at approximately 4.5 U/mL. Contains BSA and Dextran Sulfate.
Note: The BIOPHEN™ Heparin LRT (221011) and BIOPHEN™ Heparin LRT (221015) are also available.
Reagents and Materials Required but Not Provided:
The following items are recommended for use but are not provided:
- Buffers & Diluents:
- Isotonic Sample Diluent (AR034K/AR034L)
- Tris-NaCl-EDTA buffer, pH 7.85 (AR032A) / Tris-NaCl-EDTA buffer, pH 7.85 (AR032K)
- Calibrators:
- BIOPHEN™ Apixaban Calibrator (226201) / Low (226101)
- BIOPHEN™ Arixtra® Calibrator (222501)
- BIOPHEN™ Edoxaban Calibrator (226501) / Low (226401)
- BIOPHEN™ Heparin Calibrator (222001)
- BIOPHEN™ Orgaran® Calibrator (222201)
- BIOPHEN™ Rivaroxaban Plasma Calibrator (222701) / Low (226001)
- BIOPHEN™ UFH Calibrator (222301)
- Controls:
- BIOPHEN™ Apixaban Control (225301) / Low (225201)
- BIOPHEN™ Arixtra® Control Plasma (224001)
- BIOPHEN™ Edoxaban Control (225501) / Low (225401)
- BIOPHEN™ LMWH Control (223001) / (223801) / (224201) / (223701) / (224301) / (224401)
- BIOPHEN™ Orgaran® Control (223501)
- BIOPHEN™ Rivaroxaban Control Plasma (224501) / Low (225101)
- BIOPHEN™ UFH Control (223101) / (224101) / (223901)
Traceability and Performance Characteristics:
Certificates of traceability for calibrators and controls are available on the manufacturer’s website. Performance data for this assay was generated on various automated platforms.
- Measuring Range: Dependent on the analyser system used (see specific Application Guides).
- Precision: The coefficient of variation (CV) for all samples is less than 10% for repeatability, reproducibility, and within-laboratory precision.
- Interferences: No interference is expected from anti-IIa drugs. The PF4 inhibitor is limited up to 0.1 µg/mL. If the Antithrombin (AT) concentration in the tested plasma is ≤50%, levels of heparin (UFH/LMWH), Arixtra®, or Orgaran® can be underestimated.
- Agreement on ACL-TOP® Family:
- UFH (n=145): y = 1.06x + 0.08, r = 0.978 vs. HemosIL® Liquid Anti-Xa
- LMWH (n=142): y = 1.28x + 0.03, r = 0.992 vs. HemosIL® Liquid Anti-Xa
- Orgaran® (n=163): y = 1.08x – 0.01, r = 0.995 vs. HemosIL® Liquid Anti-Xa
- Arixtra® (n=153): y = 1.00x + 0.05, r = 0.992 vs. HemosIL® Liquid Anti-Xa
- Apixaban (n=150): y = 0.90x + 10.72, r = 0.994 vs. HemosIL® Liquid Anti-Xa
- Rivaroxaban (n=153): y = 1.20x + 0.66, r = 0.995 vs. HemosIL® Liquid Anti-Xa
- Agreement on STA-R® Family:
- Edoxaban (n=152): y = 0.82x + 4.11, r = 0.966 vs. STA® – Liquid Anti-Xa
- Agreement on CS-series:
- UFH/LMWH (n=182): y = 1.09x + 0.06, r = 0.959 vs. INNOVANCE® Anti-Xa
- Sensitivity/Specificity on ACL-TOP® Family:
- UFH (n=145): Sensitivity 0.939, Specificity 1.000, AUC 0.999
- LMWH (n=142): Sensitivity 0.970, Specificity 0.991, AUC 0.998
- Orgaran® (n=163): Sensitivity 0.970, Specificity 0.969, AUC 0.991
- Arixtra® (n=153): Sensitivity 0.970, Specificity 1.000, AUC 1.000
- Apixaban (n=150): Sensitivity 1.000, Specificity 0.966, AUC 0.995
- Rivaroxaban (n=153): Sensitivity 1.000, Specificity 0.996, AUC 0.996
- Sensitivity/Specificity on STA-R® Family:
- Edoxaban (n=152): Sensitivity 1.000, Specificity 0.958, AUC 1.000
Storage and Stability:
- Unopened Vials: Store at 2-8°C. Stable until the expiration date printed on the label.
- Opened Vials: Stable for 90 days at 2-8°C when stored closed and free from contamination.
Disclaimer:
The information on this page is for informational purposes and serves as a general summary. It is imperative that all users read and strictly follow the Instructions for Use (IFU) document provided with the reagent or kit they are using. This online description must not be a substitute for the official product documentation.
-
Product RangeProduct Code221013Product NameBIOPHEN™ Heparin (LRT)Product CategoryProduct BrandProduct Analyte or ApplicationProduct Size3 x 3 mlProduct Storage2°C to 8°CProduct IVD Registration StatusCE-Mark - Professional Use
UKCA -