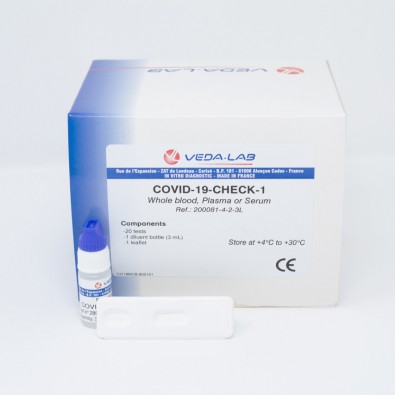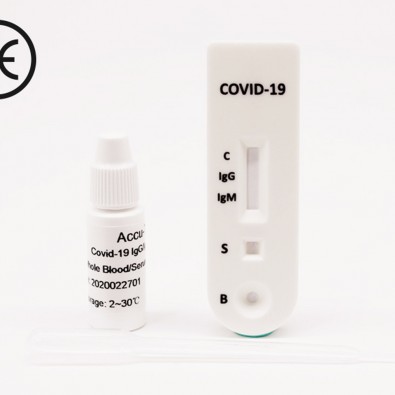

Accu-Tell SARS-CoV-2 Ag Rapid Test Cassette (CE Marked 10min COVID-19 Nasal Swab) x 20 Test Kit
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
Accu-Tell SARS-CoV-2 Ag Rapid Test Cassette (CE Marked 15min Nasopharyngeal Swab) 20 Test Kit (ABT-IDT-B367) is a rapid in vitro immunochromatographic assay lateral flow test (LFT) for the qualitative detection of SARS-CoV-2 nucleocapsid protein antigen. Manufactured by AccuBioTech for medical healthcare professional in vitro diagnostic use only.
Rapid COVID-19 Antigen Test from the manufacturer of the Accu-Tell COVID-19 IgM/IgG Antibody Test which is mentioned in an article in the Daily Mail. Following a study performed by Guy’s and St Thomas’ NHS Foundation Trust, the Accu-Tell antibody test was evaluated to be one of the most accurate on the market. The Accu-Tell Antigen Test is manufactured to the same high standards by AccuBioTech with excellent levels of specificity, sensitivity and accuracy.
For medical healthcare professional in vitro diagnostic use only
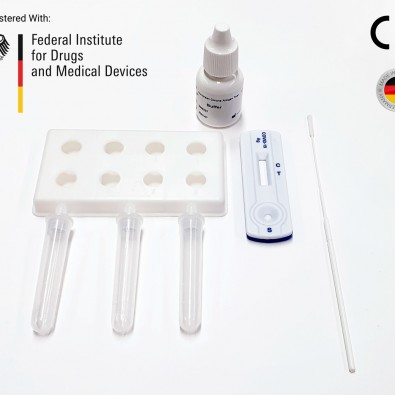
- German Government Validation: Full compliance with the Federal Institute for Drugs and Medical Devices COVID-19 testing requirements.
- Easy to Use: No special equipment needed, intuitive visual interpretation
- Rapid: Quick sampling with results in 10 min
- Convenient: All necessary reagents provided & no other equipment needed
- CE Marked: For professional use
- Accurate: 99.20% Specificity, 95.00% Sensitivity & 98.80% Accuracy
- ISO13485: AccuBioTech manufacture in accordance with their ISO13485 Quality Management accreditation. They also hold many additional ISO accreditations.
Contents:
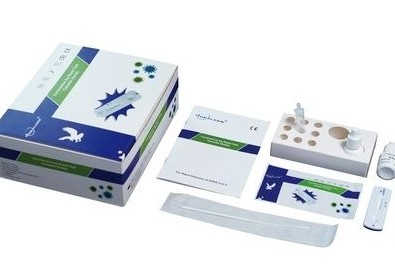
Each kit contains all the components required to run 20 tests:
- 20 Individually sealed test cassettes
- 20 Sterile swabs
- 20 Extraction tubes
- 20 Dropper tips
- 1 Extraction reagent
- 1 Workstation
- 1 Instruction manual (IFU)
Who can run these tests?
The test should be only be used in accordance with the instructions supplied and results interpreted by or under the supervision of a Medical Healthcare Professional. The test is not for home use.
What does it do?
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue, and dry cough. Nasal congestion, runny nose, sore throat, myalgia, and diarrhea are found in a few cases. This test is for the detection of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Rapid diagnosis of SARS-CoV-2 infection will help healthcare professionals to treat patients and control the disease more efficiently and effectively.
- Who can run these tests?
-
The test should be only be used in accordance with the instructions supplied by a Medical Healthcare Professional. The test is not for home use. The full Instructions for Use (IFU) document can be found HERE.
- What does the test do?
- What samples are required to run the test?
- What is the difference between Antigen testing and Antibody testing?
- What is the testing time?
- What are the possible results?
- What are the limitations of the test?
- How accurate are the tests?
- Is the test CE-marked?
- Are these tests registered with the UK Medicines and Healthcare products Regulatory Agency (MHRA)?
- Does the test need any additional components?
- Who can buy these tests?