LA Control Plasma Weak
LA Control Plasma Weak
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
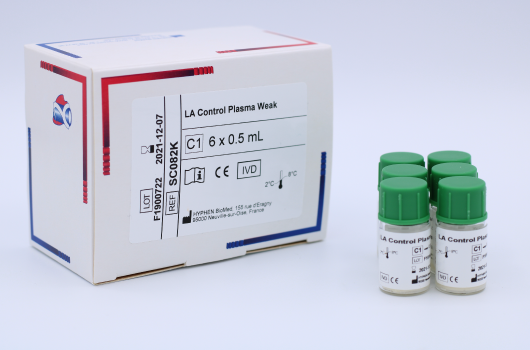
LA Control Plasma Weak (SC082K) is a set of lyophilized quality control plasmas, weak positive, for use in Lupus Anticoagulant in vitro clotting assays. Manufactured by HYPHEN BioMed.
Intended Use
LA Control Plasma Weak Kit is a set of lyophilized quality control plasmas, weak positive, for use in Lupus Ant-icoagulant in vitro clotting assays. This kit is optimized for being used with the HEMOCLOT™ LA-S and HEMOCLOT™ LA-C assays (CK090K/CK091K/CK094K).
Summary and Explanation
“Lupus anticoagulant” is associated with numerous clinical states including e.g. lupus, thrombosis, foetal loss…and must usually be confirmed from multiple assays. These control plasmas are then proposed for the quality control of Lupus anticoagulant detection in plasma using in vitro clotting assays, especially HEMOCLOT™ LA-S and HEMOCLOT™ LA-C assays (CK090K/CK091K/CK094K).
Reagents
The LA Control Plasma Weak kit contains 6 vials of 1 mL of weak positive human plasma for lupus anticoagulant.
C1 LA Control Plasma Weak. Human plasma, lyophilized.
6 vials of 1 mL.
For the assay, refer to indicative normalized ratio on the flyer provided in the kit used.
-
Product RangeProduct CodeSC082KProduct NameLA Control Plasma WeakProduct CategoryProduct BrandProduct Analyte or ApplicationProduct IVD Registration StatusCE-Mark - Professional Use
UKCAProduct Size6 x 0.5 ml -