ZYMUTEST™ HIA IgG
ZYMUTEST™ HIA IgG
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
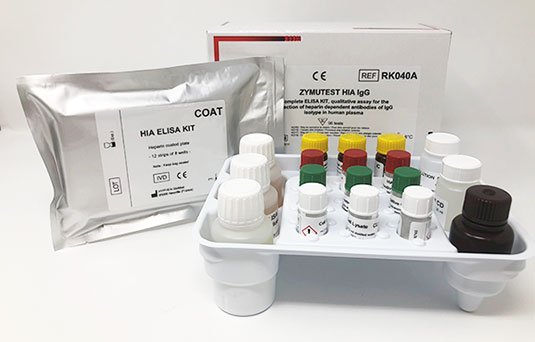
ZYMUTEST™ HIA IgG (RK040A) is an Enzyme Immuno-assay to measure Heparin-dependent antibodies of the IgG isotype in human plasma or serum. Manufactured by HYPHEN BioMed.
Enzyme Immuno-assay designed for measuring heparin-dependent antibodies of the IgG isotype, in human plasma. This assay measures only the IgG isotype, reported as the most associated with the clinical complications of heparin-dependent antibodies (HIT/HITT), allowing confirmation of the diagnosis of HIT/HITT or its clinical suspicion. However, some cases associated with only IgM and/or IgA isotypes can be missed.
Recent anecdotal evidence suggests a link between a prothrombotic state and people who have recently received a Covid vaccination. These prothrombotic states are being referred to as VATTS (vaccine-associated thrombosis/thrombocytopenia syndrome) or VIPIT (vaccine inducted prothrombotic immune thrombocytopenia). As these patients present with thrombocytopenia the diagnosis of HIT can be difficult. The ZYMUTEST™ HIA IgG ELISA has been shown to be one of the most reliable tests in identifying these individuals. Because of the design of the ZYMUTEST™ HIA IgG ELISA which uses protamine sulphate to bind the heparin and platelet factors to the micro-titre wells only patient antibodies that cause thrombocytopenia induced by heparin are detected, i.e. true hit.
ZYMUTEST™ HIA has a proven performance of many years and complies with the ISTH guidelines on HIT confirmation assays. The kits are complete with positive and negative controls, platelet lysate, wash buffer and 8 well strips. Offered in 2 formats either 12×8 wells or 4×8 wells both kits can be used for individual patient testing as the 8 well strip format can manage samples and controls thus minimising any wastage.
Overview
- CE marked kits compliant with ISTH guidelines on HIT testing
- IgG specific is one of the assays of choice for HIT confirmation
- Complete kits constructed for ease of use and economy
- 96 well and 32 well presentations for all laboratory sizes
- Manufactured to enhance binding of true HIT IgG antibodies
- Tried and tested kits available now
Product Applications
Clinical suspicion of HIT during heparin therapy.
Heparin-dependent antibodies of the IgG isotype are those strongly associated with the clinical diagnosis of HIT. The ZYMUTEST™ HIA IgG assay offers then a better specificity for the clinical complication of HIT, but it has less sensitivity as cases associated with only IgM and/or IgA isotypes are missed.
Product Characteristics
- Total Assay Time: 2 hr 15 min
- Cut Off: A450 > 0.30
- Dynamic Range: A450 up to 3.0
- Intra-Assay CV: 4 to 6%
- Inter-Assay CV: 5 to 8%
Product Components
- Micro ELISA Plate (12 strips of 8 wells)
- 2x HIA Sample Diluent.
- 3x HIA IgG Positive Control.
- 3x Negative Control.
- 3x Platelet Lysate.
- 3x Anti-IgG (Fcγ)-HRP IC.
- 1x Conjugate Diluent.
- 1x Wash Solution.
- 1x TMB.
- 1 x 0.45M Sulfuric Acid.
-
Product RangeProduct CodeRK040AProduct NameZYMUTEST™ HIA IgGProduct CategoryProduct BrandProduct Analyte or ApplicationProduct SizeKit of 96 testsProduct Storage2°C to 8°CProduct IVD Registration Status510(k),CE-Mark - Professional Use
-