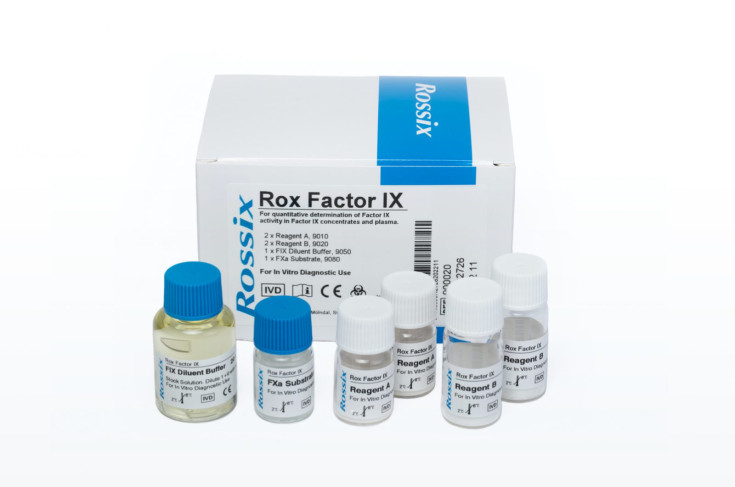
Rox Factor IX
Rox Factor IX
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
A chromogenic method for the quantitative determination of Factor IX (FIX) activity in plasma and FIX concentrates. For in vitro research use only.
Product Overview:
ROX FACTOR IX is a chromogenic method for the in vitro determination of Factor IX (FIX) activity in plasma and FIX containing concentrates. Special consultation with manufacturer recommendations and scientific literature is advised when analysing modified Factor IX molecules to ensure this assay methodology is suitable. For in vitro research use only kit.
Principle and Application:
FIX activity is determined in a chromogenic method where human FIX is activated by human Factor XIa (FXIa). The formed activated FIX (FIXa) then activates human Factor X (FX) in the presence of human Factor VIII (FVIII), calcium ions, and phospholipid. Thrombin generated during incubation activates FVIII, mimicking in vivo conditions. The amount of Factor Xa (FXa) formed is proportional to the FIX activity and is measured by the hydrolysis of a chromogenic FXa substrate. The FIX activity of the sample is determined against a plasma or concentrate standard with potency expressed in International Units (IU).
Product Composition and Specifications:
- Reagent A (2 vials, REF 9010): Lyophilized reagent containing human FVIII, human FX, bovine FV, and a fibrin polymerization inhibitor.
- Reagent B (2 vials, REF 9020): Lyophilized reagent containing human FXIa, human FII, calcium chloride, and phospholipids.
- FXa Substrate (1 vial, 6 mL, REF 9080): Liquid solution of chromogenic FXa substrate (Z-D-Arg-Gly-Arg-pNA) at 2.5 mmol/L, containing a thrombin inhibitor.
- FIX Diluent Buffer, Stock Solution (1 vial, 20 mL, REF 9050): Liquid stock solution of diluent buffer containing a heparin antagonist. Dilute 1+9 with water for the working solution.
Performance Characteristics:
- Detection Limit: 0.1% (0.001 IU/mL), calculated using the low range and a sample dilution of 1:20.
- Quantification Limit: 0.5% (0.005 IU/mL), calculated using the low range and a sample dilution of 1:20.
- Linearity: 0.5 – 200% (0.005 – 2 IU/mL), calculated according to CLSI EP06-A.
- Precision: Determined at 1%, 25%, and 100% Factor IX activity using a manual microplate method.
- Repeatability (Intra-assay CV): 3%
- Within Laboratory (Inter-assay CV): 4%
- Interference: Results are not affected by plasma concentrations up to: Hemoglobin (5 g/L), Bilirubin (0.4 g/L), Triglycerides (5 g/L), LMW Heparin (5 IU/mL), and unfractionated Heparin (2 U/mL). There is no interference of FIXa up to 50 mIU FIXa/1 IU FIX.
Storage and Stability:
- Unopened Vials: Store at 2-8°C. Stable until the expiration date printed on the label.
- Reconstituted/Opened Vials:
- Reagent A: Stable for 72 hours at 2-8°C, 8 hours at 20-25°C, or 12 months at ≤-70°C.
- Reagent B: Stable for 72 hours at 2-8°C, 8 hours at 37°C, or 12 months at ≤-70°C.
- FXa Substrate: Stable for 1 month at 2-8°C or 12 months at ≤-20°C.
- FIX Diluent Buffer Stock Solution: Stable for 12 months at 2-8°C. The prepared working solution should be used on the same day.
Disclaimer:
The information on this page is for informational purposes and serves as a general summary. It is imperative that all users read and strictly follow the Instructions for Use (IFU) document provided with the reagent or kit they are using. This online description must not be a substitute for the official product documentation.
-
Product RangeProduct Code900020Product NameRox Factor IXProduct CategoryProduct BrandProduct Analyte or ApplicationProduct Size2 x 50 testsProduct Storage2°C to 8°CProduct IVD Registration StatusCE-Mark - Professional Use
UKCA