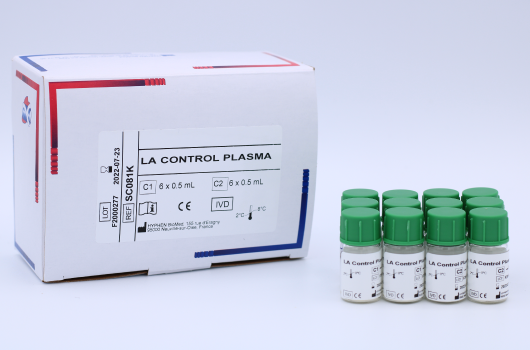
LA Control Plasma
LA Control Plasma
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
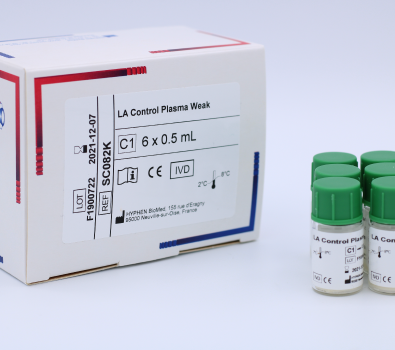
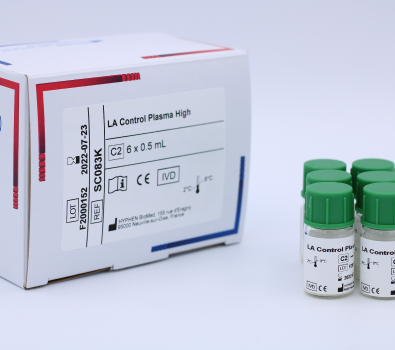
LA Control Plasma (SC081K) is a set of lyophilized quality control plasmas, weak and high positive, for use in Lupus Anticoagulant clotting assays. Manufactured by HYPHEN BioMed.
Intended Use
LA Positive Control Plasma Kit is a set of lyophilized quality control plasmas, weak and high positive, for use in Lupus Anticoagulant in vitro clotting assays.
This kit is optimized for being used with the HEMOCLOT™ LA-S and HEMOCLOT™ LA-C assays (CK090K/CK091K/CK094K).
Summary and Explanation
“Lupus anticoagulant” is associated with numerous clinical states including eg lupus, thrombosis, foetal loss…and must usually be confirmed from multiple assays. These control plasmas are then proposed for the quality control of Lupus anticoagulant detection in plasma using in vitro clotting assays, especially HEMOCLOT™ LA-S and HEMOCLOT™ LA-C assays (CK090K/CK091K/CK094K).
Reagents
The LA CONTROL PLASMA kit contains 12 vials of 1 mL of positive human plasma for lupus anticoagulant at two different levels (6 vials for each level).
C1 LA Control Plasma Weak. Human plasma, lyophilized, 6 vials of 1 mL.
C2 LA Control Plasma High. Human plasma, lyophilized, 6 vials of 1 mL.
For the assay, refer to indicative normalized ratio on the flyer provided in the kit used.
-
Product RangeProduct CodeSC081KProduct NameLA Control PlasmaProduct CategoryProduct BrandProduct Analyte or ApplicationProduct IVD Registration StatusCE-Mark - Professional Use
UKCAProduct Size2 x 6 x 0.5 ml -