BIOPHEN™ Bivalirudin Control
BIOPHEN™ Bivalirudin Control
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
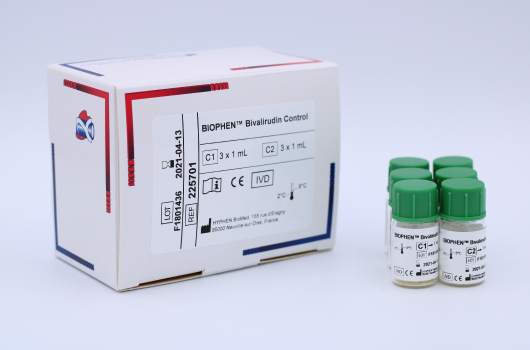
BIOPHEN™ Bivalirudin Control (225701) is a kit used for the quality control of Bivalirudin assays in plasma. Manufactured by HYPHEN BioMed.
Intended Use
The BIOPHEN™ Bivalirudin Control kit consists of lyophilized human plasmas, spiked with Bivalirudin at various concentrations, for the quality control of Bivalirudin assays.
They are titrated and optimized for the assay of Bivalirudin by anti-IIa clotting and chromogenic techniques.
Summary and Explanation
Technical These controls are proposed for the quality control of anti-IIa clotting and chromogenic assays of Bivalirudin in plasma (HEMOCLOT™ Thrombin Inhibitors and BIOPHEN™ DTI).
Clinical Bivalirudin can be used as an anticoagulant for curative indications, mainly in emergency situations. Measuring the Bivalirudin concentration in patients’ plasma can be used for monitoring the therapy and adjusting drug dosage.
Reagents
C1 Control 1: Lyophilized human plasma containing a titrated quantity of Bivalirudin of approximately 1.50 µg/mL. C2 Control 2: Lyophilized human plasma containing a titrated quantity of Bivalirudin of approximately 4.00 µg/mL.
Each kit contains 3 vials of 1 mL of C1 and 3 vials of 1 mL of C2.
The control plasmas contain stabilizing agents.
The control concentrations may vary slightly from one batch to another. For the assay, see the exact values indicated on the flyer provided with the kit used.
-
Product RangeProduct Code225701Product NameBIOPHEN™ Bivalirudin ControlProduct CategoryProduct BrandProduct Analyte or ApplicationProduct IVD Registration StatusCE-Mark - Professional Use
UKCAProduct Size2 x 3 x 1 mlProduct Storage2°C to 8°C -