News
All the latest news from Quadratech Diagnostics. If you would like to receive updates via our newsletter then please sign up here.
2025 Christmas and New Year Shipping Details
As Christmas and the New Year are approaching rapidly we would would like to inform our customer of our amended operating hours. This year we will be closing our office for the holidays on 12:00 noon Wednesday 24/12/25 until Monday 05/01/26. For International shipments: The last normal shipping day is Monday 15/12/25 with normal service […]
Haemostasis Q4 2025 Journal Club
Keep informed about evolving science and clinical practice with our compilation of the latest studies, guidelines, and perspectives in haemostasis. GUIDELINES Jennings I, Baker P, Bowyer A, et al. Reporting haemostasis assays in IU/dL-A recommendation for harmonisation from the British Society for Haematology Haemostasis and Thrombosis Task Force, UK NEQAS for Blood Coagulation and the […]
Haemostasis Q4 2025 News
Our round-up of the latest developments from across the haemostasis industry for Q4 2025. REGULATORY TAHO Pharma submitted the New Drug Application to the FDA for TAH3311 (apixaban oral dissolving film), developed on its proprietary Transepithelial Delivery System (1 Oct) ADLM (Association for Diagnostics & Laboratory Medicine) released new guidance on coagulation testing in patients […]
Harnessing bacteria’s potential: Cholera Toxin Products
Harnessing bacteria’s potential: Cholera Toxin Products Cholera toxin is produced by Vibrio cholerae, a gram-negative bacterium, and is the main virulence factor in the development of cholera, a waterborne acute diarrheal disease. As a classic AB Type toxin, cholera toxin consists of a single A subunit surrounded by five identical B subunits, arranged as pentamer […]
UK NEQAS for Laboratory and Clinical Haemostasis 2025 Reflections
UK NEQAS for Laboratory and Clinical Haemostasis 2025: Key Takeaways and Reflections by Quadratech Diagnostics Team In June, we were delighted to sponsor UK NEQAS Blood Coagulation Annual Scientific Meeting in Sheffield. We truly valued the opportunity to meet with our existing customers and to welcome new participants interested in our diagnostic solutions. The two-day […]
Haemostasis Q3 2025 Journal Club
Keep informed about evolving science and clinical practice with our compilation of the latest studies, guidelines, and perspectives in haemostasis. GUIDELINES Barrett WJ, Kaucher KA, Orpet RE, et al. Tranexamic acid in trauma: A joint position statement and resource document of NAEMSP, ACEP, and ACS-COT. J Trauma Acute Care Surg. 2025;99(3):357-363. doi:10.1097/TA.0000000000004727 Lier H, Goossen K, […]
Haemostasis Q3 2025 News
Our round-up of the latest developments from across the haemostasis industry for Q3 2025. REGULATORY Novo Nordisk’s Alhemo (concizumab-mtci, a tissue factor pathway inhibitor antagonist) injection received positive opinion by the EMA CHMP, recommending label expansion to include the treatment of severe haemophilia A and moderate or severe haemophilia B without inhibitors (25 Jul) Kedrion […]
Haemostasis Q2 2025 News
Our round-up of the latest developments from across the haemostasis industry for Q2 2025, including the latest data presentations at ISTH 2025. REGULATORY FDA approved Bayer’s Jivi (a recombinant extended half-life factor VIII concentrate) for use in paediatric patients 7 years of age and older with haemophilia A based on Alfa-PROTECT and PROTECT Kids trial […]
Virus-like particles for vaccine R&D and diagnostic assays
Virus-like particles (VLPs) are self-assembled protein nanoparticles that closely resemble viruses, but contain no viral genetic material and are replication-deficient. First discovered in 1968 in sera of patients infected with Hepatitis B virus, VLPs have since become vital tools for scientific and translational research in biology and medicine. The utility of VLP technology has expanded […]
Infectious Diseases News – Spring 2025
Our round-up of the latest developments from across infectious disease research and prevention in Spring 2025. DIAGNOSTICS The WHO published its first analysis of commercially available and pipeline diagnostic products for invasive fungal diseases caused by WHO Fungal Priority Pathogens and the identifiable gaps.(19 Mar) The WHO issued the information notice for users of malaria […]
Come Meet Us at the UK NEQAS Blood Coagulation ASM at Stand 2!
COME MEET US We are delighted to announce we will be a proud sponsor and exhibitor at the upcoming UK NEQAS Blood Coagulation ASM (Annual Scientific Meeting) in Sheffield. We very much look forward to meeting those in attendance and will be happy to discuss our offering of diagnostic reagents and kits for the […]
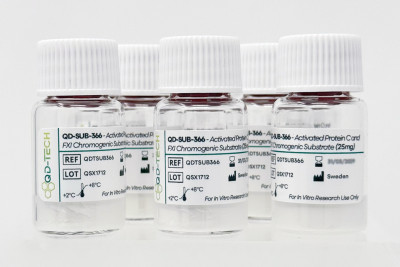
Quadratech Diagnostics Launches QD-SUB-366 – Activated Protein C and FXI Chromogenic Substrate
We are delighted to announce the global launch of QD-SUB-366 – Activated Protein C and FXI Chromogenic Substrate, the first chromogenic substrate from our new QD-TECH range. Developed through close collaboration with leading scientists, clinicians, and ISO/FDA-registered European manufacturers, QD-TECH reagents are tailored for use in clinical laboratories, research centres, and the pharmaceutical industry. QD-SUB-366 […]