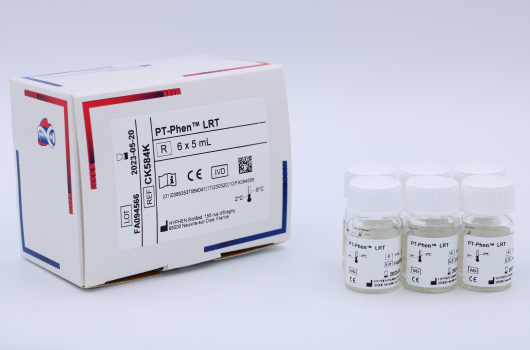
PT-Phen™ LRT - 6 x 5 ml
PT-Phen™ LRT – 6 x 5 ml
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
PT-Phen™ LRT (CK584K) is a clotting method for the in vitro quantitative determination of Prothrombin Time (PT), in human citrated plasma, with ready-to-use liquid reagents. Manufactured by HYPHEN BioMed.
Intended Use
Clotting method for the in vitro quantitative determination of Prothrombin Time (PT), in human citrated plasma, using an automated method. This global method is for the screening of the coagulation disorders (extrinsic pathway) and the monitoring of Vitamin K Antagonists (VKAs) treatments. This therapy is currently used for curative or preventive indications of thrombotic diseases.
This device of in vitro diagnostic use is intended for professional use in the laboratory.
Summary and Explanation
Technical:¹˒² PT is a one-stage test based on the Quick method. PT assesses the overall activity of clotting factors of the extrinsic pathway (coagulation triggered by contact with thromboplastin expressed by specific cells). PT does not explore factor deficiencies of the intrinsic coagulation pathway (Factors VIII, IX, XI, and XII), platelets, Factor XIII or natural inhibitors of coagulation (Antithrombin, Protein C and Protein S).
Clinical:²˒³ The PT test is used to explore abnormalities of factors II, V, VII, X and fibrinogen (Fib) in bleeding disorders, liver disease, vitamin K deficiency, disorders of fibrinolysis and disseminated intravascular coagulation (DIC). PT test is the most commonly used coagulation assay for laboratory monitoring of anticoagulant therapy by VKAs. Any isolated prolongation of PT, not associated with VKA therapy, has to be explored by individual assay of factors involved in the extrinsic coagulation pathway (FII, FVII, FV, FX).
Principle
PT is the determination of a clotting time at 37°C in the presence of thromboplastin and calcium, by initiation of activation of the extrinsic coagulation pathway (complex TF-FVIIa activated factor X, then generation of thrombin, leading to fibrin formation). The clotting time depends on the activity of extrinsic pathway coagulation factors (II, V, VII, X, Fib). Measured PT can be converted to concentration (PT%), or expressed as a ratio (PT ratio), or as recommended “International Normalized Ratio (INR)”.
The reagent includes a heparin neutralizing substance.
-
Product RangeProduct CodeCK584KProduct NamePT-Phen™ LRT – 6 x 5 mlProduct CategoryProduct BrandProduct Analyte or ApplicationProduct Size6 x 5 mlProduct Storage2°C to 8°CProduct IVD Registration StatusCE-Mark - Professional Use
UKCA -