BIOPHEN™ V-L Control Plasma
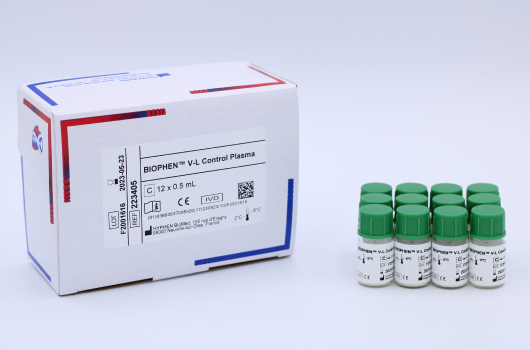
BIOPHEN™ V-L Control Plasma
We're always working hard to give our customers as much information on products and the best price possible. If you need any assistance or would like a quote please contact us and we will be happy to help
-
Product Summary:
BIOPHEN™ V-L Control Plasma (223405) is a pathological human plasma for the quality control of Factor V-Leiden assays by clotting method. Manufactured by HYPHEN BioMed.
Intended Use
The BIOPHEN™ V-L Control Plasma kit consists of undiluted lyophilized human plasma, titrated at one concentration of Factor V-Leiden (FV-L), for the quality control of FV-L assay.
It is titrated and optimized for the quantitative assay of FV-L by clotting method.
Summary and Explanation
Technical Activated Protein C plays a role of regulator in the coagulation process by specifically inactivating activated Factors V (Va) and VIII (VIIIa), in the presence of co-factors. The resistance phenomenon to activated Protein C (APC) is due in more than 90% of cases to the R506Q mutation of Factor V called “Factor V Leiden”. This mutation in Factor V exon 10 (1691 G → A) substitutes arginine at position 506 by glutamine, prevents this site cleavage by APC.
This control is proposed for the quality control of clotting assay of FV-L in plasma (HEMOCLOT™ Quanti. VL).
Clinical FV-L mutation is the most common hereditary thrombophilia risk factor. Its prevalence is about 5% in Caucasian populations. Patients carrying FV-L mutation have an increased risk of venous thrombosis, 3 to 7 fold in heterozygotes and up to 80 fold in homozygotes.
This genetic anomaly can be evidenced by clotting assay in the presence or absence of APC.
Reagents
C Control: Undiluted lyophilized human plasma, containing a titrated quantity of FV-L of approximately 40% (plasma presenting APC resistance).
12 vials of 0.5 mL.
Control plasmas contain stabilizing agents.
The control concentrations may vary slightly from one batch to another. For the assay, see the exact values indicated on the flyer provided with the kit used.
-
Product RangeProduct Code223405Product NameBIOPHEN™ V-L Control PlasmaProduct CategoryProduct BrandProduct Analyte or ApplicationProduct Size12 x 0.5 mlProduct Storage2°C to 8°CProduct IVD Registration Status510(k)
CE-Mark - Professional Use
UKCA -